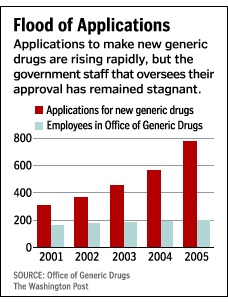
In my last post about Seroquel’s future, I mentioned the Hatch-Waxman Act (PDF), which was passed in 1984. The Act was supposed to speed up the adoption of generic drugs when the patents behind name-brand drugs ran out. This happened at first, but as pharmacy has expanded, the Act has created a bottleneck at the FDA. It is speculated that the bottleneck is Big Pharma itself: creative lobbying seems to have resulted in a reduction in the budget for the Office of Generic Drugs — which in turn has limited its capacity to approve generic drugs to some 400 per year.
Unfortunately, I can’t seem to find which drugs’ patents will expire without a generic equivalent to take its place — I suspect that none of them are massively profitable by themselves — but all told, the market value of these 800 drugs is a whopping $78 billion per year for their manufacturers. For comparison, the entire generic drug industry is only worth just over $22 billion — even though it accounts for over half the prescriptions dispensed each year in the United States. Broken down, that’s almost $100 million per drug, ceteris paribus. Regardless of the specifics of each case, the aggregate dollars involved are huge.
If a creative generic manufacturer like Teva can squeak a few generic approvals by, they will effectively have a six-month monopoly on those drugs. The Hatch-Waxman Act allows the first generic manufacturer to produce and sell their generic equivalent with no competition — save from its brand equivalent. These first six months are crucial. During this time, a generic drug usually undercuts the brand-name competition by about 6%. This is a hugely profitable time, because once other generic manufacturers are allowed to start selling their own equivalents, the price drops to some 80% less than the brand-name drug, so in theory, that first-to-market manufacturer can make more in those first six months than they could in the 4-5 years after their monopoly ends.
Big Pharma sometimes combats this by paying off the first generic manufacturer to continue their monopoly for another 180 days — which in the case of a huge drug like Zoloft or Zocor could be in the billions of dollars of additional revenue. And sometimes they roll out a lower priced “generic” version themselves, which is really just the name-brand med without the name. (One of these days I’ll give you an example of how convoluted — and silly — the whole brand-generic wars can be. It’s actually kind of funny, but with billions of dollars in revenue at stake, it’s a game well-worth playing.)
[tags]patent protection, FDA, generic drugs, pharmacy[/tags]
5 thoughts on “800 drug patents backlogged at the FDA”